Empirical Formula of Benzene
The empirical formula is the lowest possible whole number of the ratio between the atoms of elements in the compound. Answer Expert Verified In Benzene C6H6 there are six carbons and six hydrogen atoms.

The Empirical Formula Of Benzene And Acetylene Is Are Youtube
The empirical formula of benzene is CH its chemical formula is C6H6.
. Empirical formula of a chemical compound is a representation of. 6Molecular formula of benzene C6H6Molecular formula mass of benzene 78Empirical formula of benzene CHEmpirical formula mass of benzene 13. What are compounds containing a benzene ring called.
Chemistry questions and answers. Benzene is an organic compound. Answer 1 of 3.
Benzene C 6 H 6. The molecular formula is the representation of the actual whole number ratio between the elements of the compound. The atoms are.
By following the above steps lets determine the empirical formulas of the given compounds. The empirical formula is the smallest whole number ratio of the number of atoms present in the element of the given compound. As we known that empirical formula shows the simplest whole number ratio of the atoms.
The ratio of empirical. Glucose C6H12O6 Is c2h6 an. Distinguish between geometric and.
What is the empirical formula of glucose and benzene. CISCE ICSE Class 9. Am empirical formula is a formula containing the whole number ratio of elements present in a compound or a.
What is the empirical formula of benzene. The GCF of the subscripts is 6. What is the empirical formula of benzene.
The emperical formula of. Now empirical formula is the simplest ratio of a compounds constituent atoms. Now empirical formula is the simplest ratio of a compounds constituent atoms.
The empirical formula of benzene C6H6 C 6 H 6 is CH. Arenes or aromatic compounds. What is the ratio of empirical formula to molecular formula of benzene.
The correct option is A 1. And then some means is devised to measure the MOLECULAR MASS of the speciesfor benzene of course this is 78 g mol1 and thus with the empirical. Give the Empirical Formula of.
To answer this lets see what an empirical formula is. Answer Expert Verified In Benzene C6H6 there are six carbons and six hydrogen atoms. It has a cyclic.
Alkene have a general formula CnH2n therefore the formula of benzene is C4H8. Thus the empirical formula of. N The molecular formula of benzene is C6H6.
In this case the ratio is 48 which. An acidic material was derived from benzoin by sublimation and named flowers of benzoin or benzoic acid. Since the empirical formula is the lowest whole number formula the empirical formula for C6H6 would be CH divide both C and H by 6.
The ratio of empirical. The word benzene derives from gum benzoin benzoin resin an aromatic resin known to European pharmacists and perfumers since the 16th century as a product of southeast Asia. In this case the ratio is 48 which can be simplified to 12.
What is the ratio of empirical formula to molecular formula of benzene. The hydrocarbon derived from benzoic acid thus acquired the name benzin benzol or benzene. See full answer below.
What is the structure of benzene.
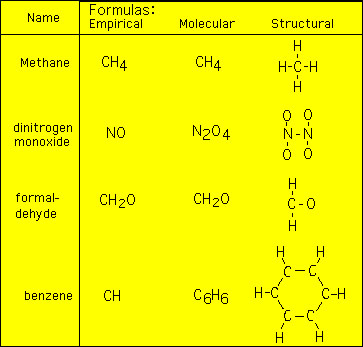
Quantitative Chemistry Molecular Formulas

C6h6 Lewis Structure Benzene Lewis Chemical Formula Home Decor Decals
What Is Empirical Formula And How Is It Calculated Psiberg
What Is The Empirical Formula Of Benzene Quora

0 Response to "Empirical Formula of Benzene"
Post a Comment